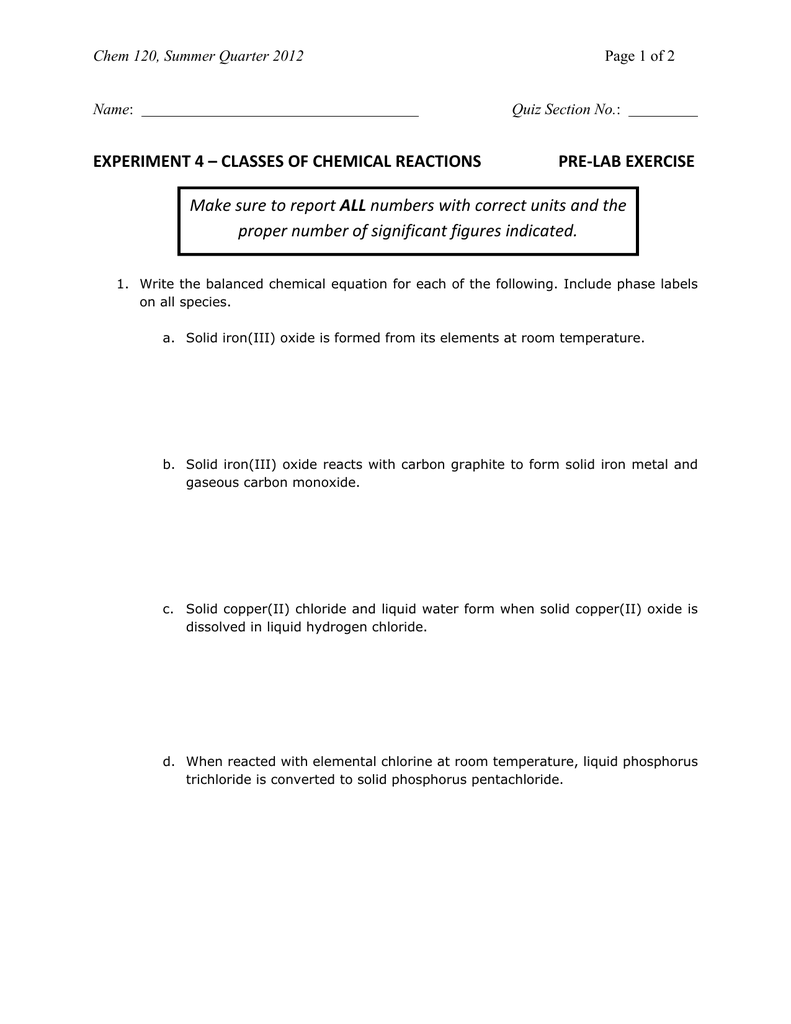
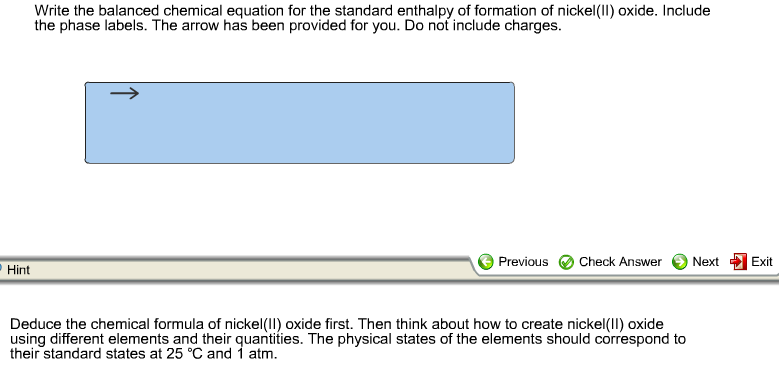
45 phase labels in chemical equations
Regional Screening Levels (RSLs) - What's New | US EPA Web18.05.2022 · The labels for adult and child body weight in the Calculator output were corrected. The target risk labels in the Resident Air Supporting Table were corrected. The Inhalation Unit Risk for TCDD was changed to 38 and the Generic Tables were updated. For assistance/questions please use the Regional Screening Levels (RSLs) contact us page. 1.6 Mathematical Treatment of Measurement Results Web4.1 Writing and Balancing Chemical Equations; 4.2 Classifying Chemical Reactions; 4.3 Reaction Stoichiometry; 4.4 Reaction Yields; 4.5 ... 10.4 Phase Diagrams; 10.5 The Solid State of Matter; 10.6 Lattice Structures ... approach is the same—all the factors involved in the calculation must be appropriately oriented to ensure that their labels ...
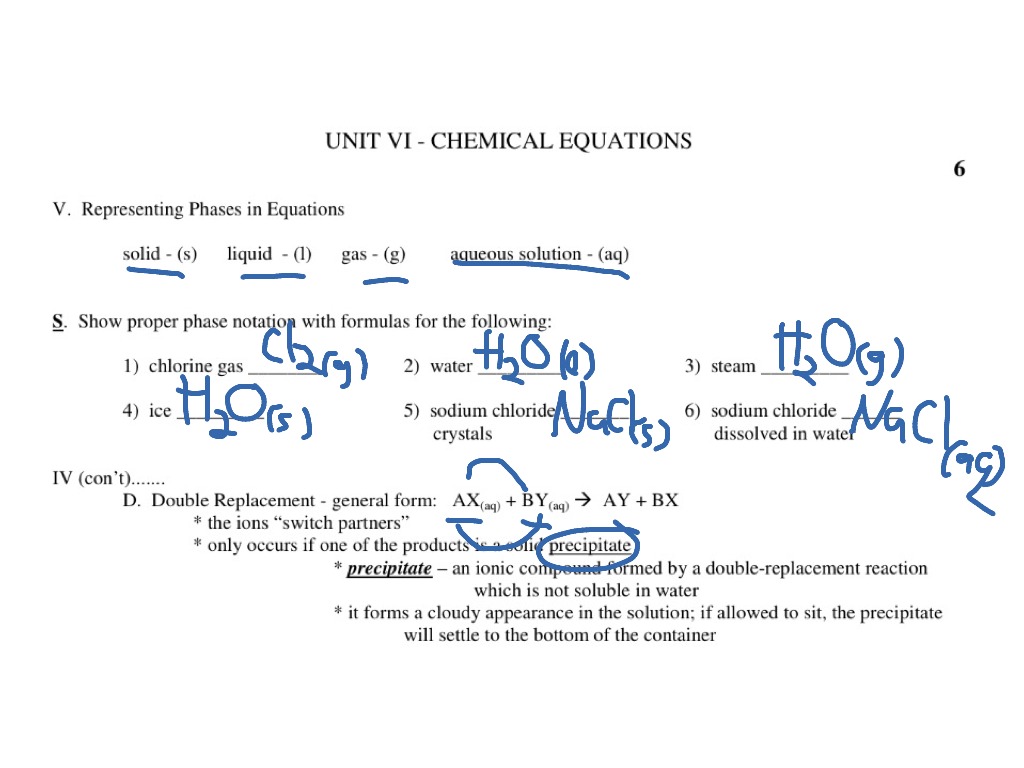
chemical equation | Yeah Chemistry Formulas of the reactants appear on the left side of the equation; those of products are written on the right. In many cases it is useful to indicate the state or phases of the substance in an equation.You use the following phase labels: (g) = gas , (l) = liquid , (s) = solid , (aq) = water solution
Phase labels in chemical equations
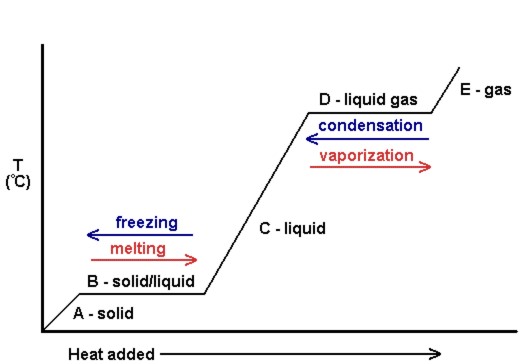
Phase Transitions: Melting, Boiling, and Subliming Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows: H 2 O (s) → H 2 O (ℓ) No chemical change is taking place; however, a physical change is taking place. Heating Curves Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust. Laboratory evaluation of alum, ferric and ferrous-water treatment ... Web27.08.2020 · After the adsorption phase qe's were calculated , the data were fitted to determine the kinetic coefficients. This was only possible for the adsorption phase as desorption phase with surface water introduced new PO 4 at each refill step. The data was evaluated with both the Langmuir and Freundlich equations models.
Phase labels in chemical equations. 6.3 Acid-Base Reactions - CHEM 1114 - Introduction to ... - BCcampus According to the solubility rules, Ca 3 (PO 4) 2 is insoluble, so it has an (s) phase label. To balance this equation, we need two phosphate ions and three calcium ions; we end up with six water molecules to balance the equation: 2 H3PO4(aq) + 3 Ca (OH)2(aq) 6 H2O (ℓ) + Ca3(PO4)2(s) This chemical equation is now balanced. Test Yourself PDF Chemical reaction : conversion of substances into different substances ... Phase labels : show the phase of reactants or products (s) : (l) : (g) : (aq ) : Chapter 7: Chemical Reactions ch7a Page 1 ___ H 2+ ___ O 2 → ___ H 2O Law of conservation of mass: 1. Leave elemental substances for last: ... Use the chemical equations worksheet to practice writing and balancing chemical equations. Chapter 4 Section E Neutralization Reactions - PEOI Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. For example, the chemical reaction between HCl(aq) and Fe(OH) 3 (s) still proceeds according to the equation 3HCl(aq) +Fe(OH) 3 (s) --> 3H 2 O(l) +FeCl 3 (aq) even though Fe(OH) 3 is not soluble. When one realizes that Fe(OH) 3 (s) is a component of rust, this explains why ... Common wrong-answer feedback for chemical formula answers - pearsoncmg.com When stacked super/sub script is used for phases or when a phase is placed before an ion charge, give the unpenalized response "Check your placement of subscripts and superscripts. Place phases at the end of a chemical formula." ... give the unpenalized response "Check the reaction arrow in your chemical equation." See also: Chemical formula or ...
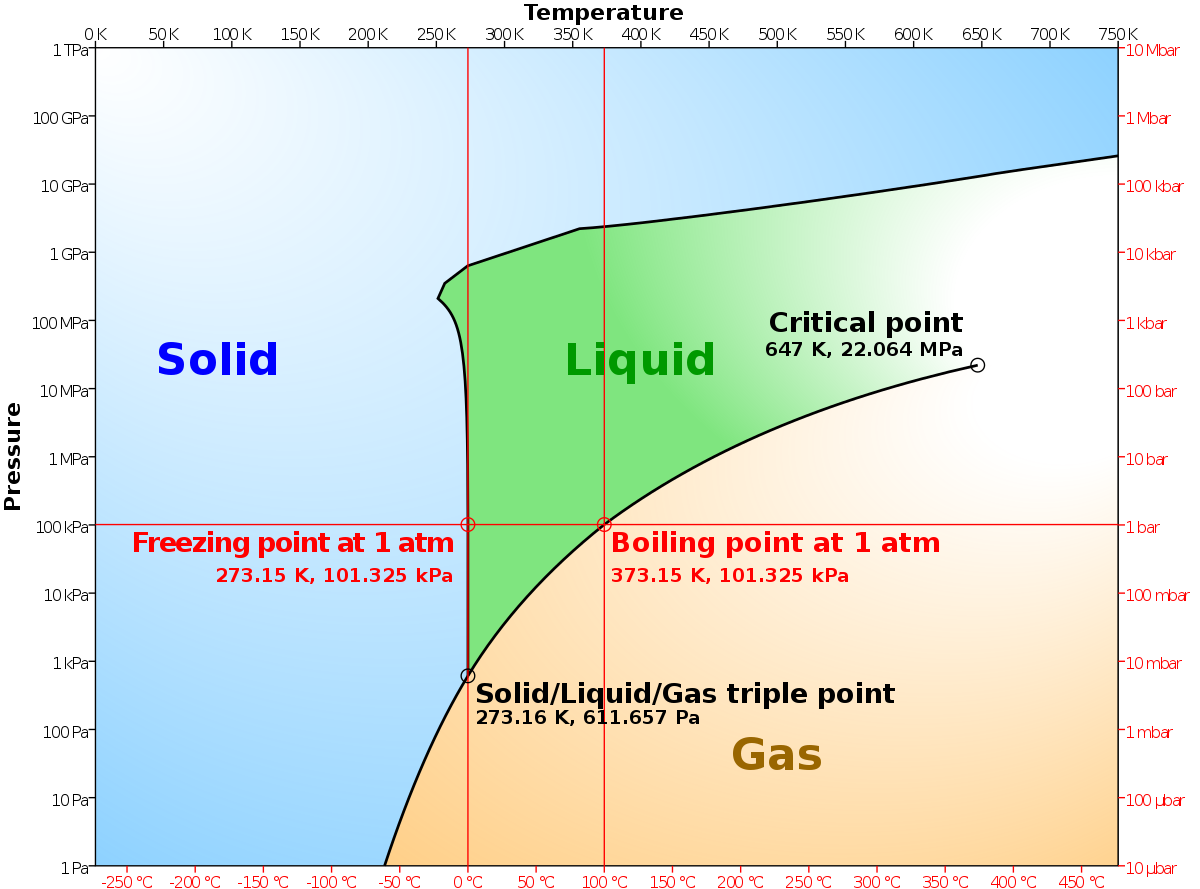
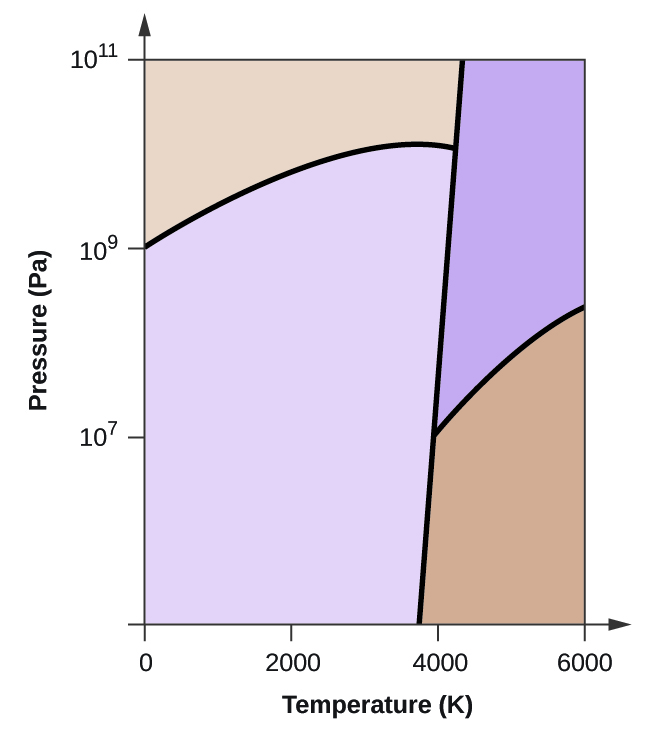
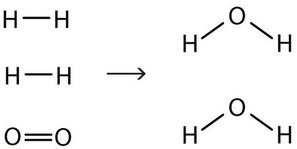
End-of-Chapter Material - Introductory Chemistry- 1st Canadian Edition Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4 Na (s) + 2 Cl2(g) → 4 NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2 O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Phase diagram - Wikipedia The simplest phase diagrams are pressure-temperature diagrams of a single simple substance, such as water.The axes correspond to the pressure and temperature.The phase diagram shows, in pressure-temperature space, the lines of equilibrium or phase boundaries between the three phases of solid, liquid, and gas.. The curves on the phase diagram show the points where the free energy (and other ... Examples of Balanced Chemical Equations - ThoughtCo 6 CO 2 + 6 H 2 O → C 6 H 12 O 6 + 6 O 2 (balanced equation for photosynthesis) 6 carbon dioxide + 6 water yields 1 glucose + 6 oxygen. 2 AgI + Na 2 S → Ag 2 S + 2 NaI. 2 silver iodide + 1 sodium sulfide yields 1 silver sulfide + 2 sodium iodide. Ba 3 N 2 + 6 H 2 O → 3 Ba (OH) 2 + 2 NH 3. The Chemical Equation – Introductory Chemistry – 1st Canadian ... Write and balance the chemical equation that represents nitrogen and hydrogen reacting to produce ammonia, NH 3. Answer N 2 + 3H 2 → 2NH 3 Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water).
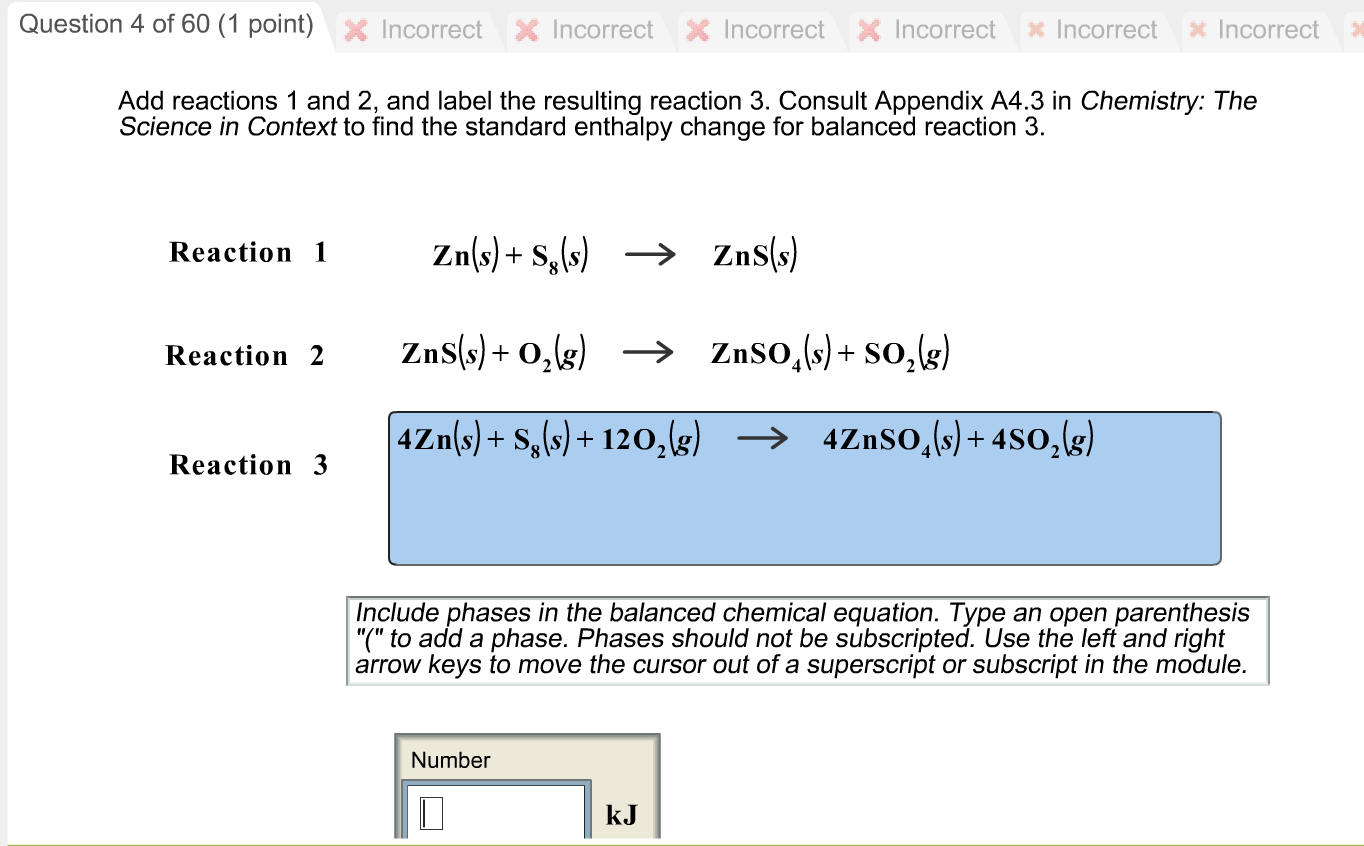
A thermochemical equation is a balanced chemical reaction equation ... A thermochemical equation is a balanced chemical reaction equation (including phase labels) with the enthalpy of reaction value written directly after the equation THERMOCHEMICAL EQUATIONS Many chemical reactions are accompanied by heat. Some reactions produce heat, while others require heat for the reaction to occur. Neutralization Reactions - GitHub Pages Neutralization reactions are one type of chemical reaction that proceeds even if one reactant is not in the aqueous phase. For example, the chemical reaction between HCl(aq) and Fe(OH) 3 (s) still proceeds according to the equation 3HCl(aq) + Fe(OH) 3 (s) → 3H 2 O(ℓ) + FeCl 3 (aq) even though Fe(OH) 3 is not soluble. When one realizes that Fe(OH) 3 (s) is a component of rust, this explains ... End-of-Chapter Material Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels. Explain why 4Na (s) + 2Cl2(g) → 4NaCl (s) should not be considered a proper chemical equation. Explain why H2(g) + 1/2O2(g) → H2O (ℓ) should not be considered a proper chemical equation. Scientific law - Wikipedia WebScientific laws or laws of science are statements, based on repeated experiments or observations, that describe or predict a range of natural phenomena. The term law has diverse usage in many cases (approximate, accurate, broad, or narrow) across all fields of natural science (physics, chemistry, astronomy, geoscience, biology).Laws are developed …
Definition of dissolve and the state label (aq) in chemical equations $\ce{CO2 (aq)}$would imply dissolved carbon dioxide in water (i.e.,to our eyes it is a single phase, just like an unopened bottle of coke). $\ce{Br2 (aq)}$would imply homogeneous solution of liquid bromine in water. $\ce{NaCl (aq)}$: Salt dissolved in water. A colloid is a heterogeneous phase.
Phase Diagrams - Chemistry - University of Hawaiʻi Using the phase diagram for water given in [link], determine the state of water at the following temperatures and pressures: (a) −10 °C and 50 kPa (b) 25 °C and 90 kPa (c) 50 °C and 40 kPa (d) 80 °C and 5 kPa (e) −10 °C and 0.3 kPa (f) 50 °C and 0.3 kPa Solution
Chapter 5 - Chemical Reactions and Equations - CHE 105/110 ... Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, 2NaHCO3 (s) − →−200°C Na2CO3 (s) +CO2(g) +H2O(ℓ) Key Takeaways
The Chemical Equation - GitHub Pages Many chemical equations also include phase labels for the substances: (s) for solid, (ℓ) for liquid, (g) for gas, and (aq) for aqueous (i.e., dissolved in water). Special conditions, such as temperature, may also be listed above the arrow. For example, Key Takeaways A chemical equation is a concise description of a chemical reaction.
EEP - Electrical Engineering Portal | Energy and Power For All Web19.09.2022 · Our mission is to be the leading provider of scientific information in the field of power and engineering in general. We publish, we share and we spread the knowledge. You're welcome to read, write and contribute to EEP in any way!
Phase Rule - Teaching Phase Equilibria The system is entirely composed of H 2 O, so there is only one component present.; The phases present represent three states of matter: liquid (water), solid (ice), and vapor (steam). All have distinct physical properties (e.g. density, structure--or lack of, etc.) and chemical properties (e.g. Δ G formation, molar volume etc.) so they must be considered distinct phases.
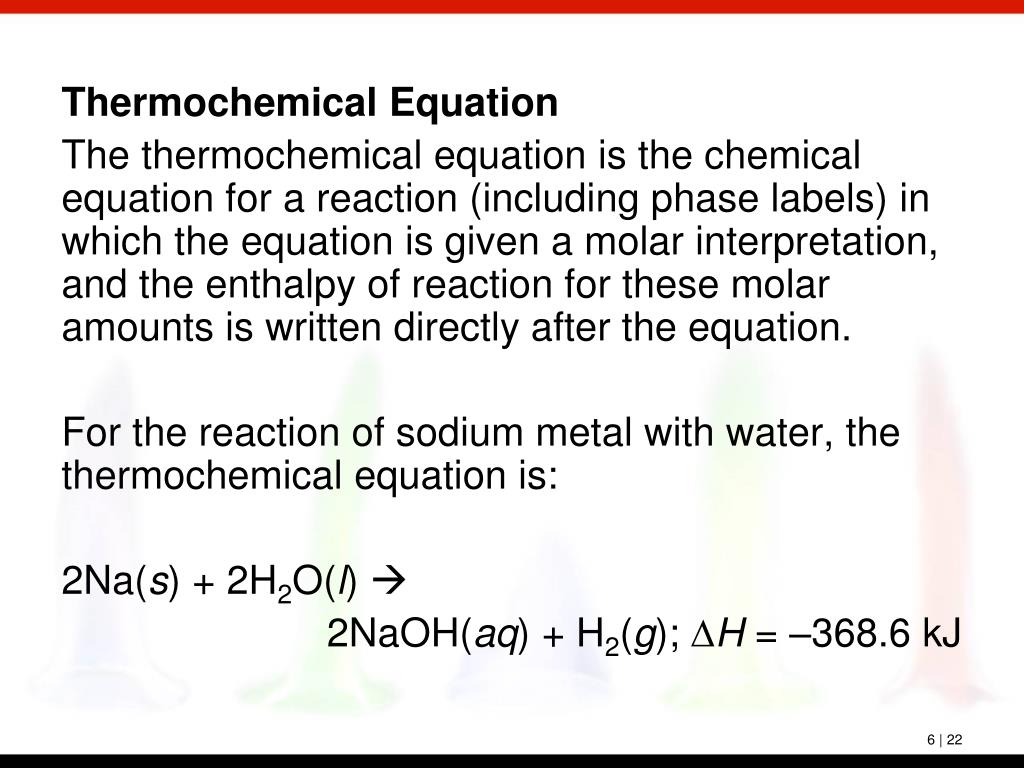
Molar Interpretation of a Chemical Equation - Big Chemical Encyclopedia Thermochemical equation the chemical equation for a reaction (including phase labels) in which the equation is given a molar interpretation, and the enthalpy of reaction for these molar amounts is written directly after the equation. (6.4) Thermochemistry the study of the quantity of heat absorbed or evolved
Lagrangian mechanics - Wikipedia Webapplies to each particle. For an N particle system in 3 dimensions, there are 3N second order ordinary differential equations in the positions of the particles to solve for.. The Lagrangian. Instead of forces, Lagrangian mechanics uses the energies in the system. The central quantity of Lagrangian mechanics is the Lagrangian, a function which …
Chapter 7 The chemical equation that can be written to describe the solubility of sodium chloride is, NaCl (s)---H 2 O--> Na + (aq) + Cl-(aq) We can write the equation to describe what happens when copper(II) chloride dissolves in water, ... Write the formula and identify the phase for the product(s) and balance the following reaction. Na 2 SO 4(aq ...
End-of-Chapter Material | Introductory Chemistry Chemical equations can also be used to represent physical processes. Write a chemical reaction for the freezing of water, including the proper phase labels.
State symbols and phase changes | StudyPug The phase can affect how reactive a substance is, but changing phase (a physical change) is not the same as changing the substance (a chemical change). A fully detailed chemical equation will show the state (or phase) of matter that the atoms or molecules are in. These states are: Solid, given the symbol (s) Liquid, given the symbol (l)
Solved 1. Write a balanced thermochemical equation with | Chegg.com Question: 1. Write a balanced thermochemical equation with phase labels for the Haber process with the heat energy as part of the equation. (3 pts) 2. What is the theoretical yield of ammonia (in grams) if 16.55 grams of nitrogen gas and 10.15 grams of hydrogen gas are allowed to react? (9 pts) 3.
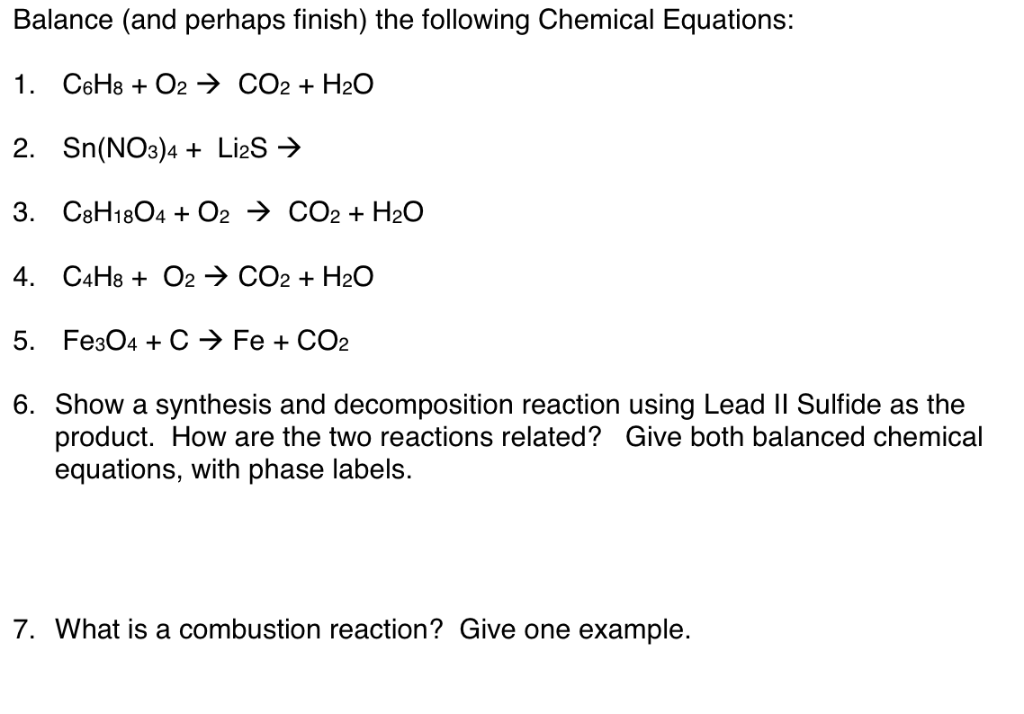
3 Steps for Balancing Chemical Equations - ThoughtCo To do this, you need to be familiar with the properties of various compounds or you need to be told what the phases are for the chemicals in the reaction. Oxides are solids, hydrogen forms a diatomic gas, tin is a solid, and the term ' water vapor ' indicates that water is in the gas phase: SnO 2 (s) + 2 H 2 (g) → Sn (s) + 2 H 2 O (g)
Electrochemcal Impedance Spectroscopy (EIS) Basics Web27.09.2022 · The impedance is proportional to the frequency-dependent voltage and frequency-dependent current , where is the angular frequency of an oscillating sine wave. The definition of impedance comes from electrical circuits, and as a result, voltage is commonly used to define impedance. However, in electrochemical impedance …
Thermochemical equation for the decomposition of potassium chlorate has ... The chemical equation in which the equation is given a molar interpretation with phase labels and also the enthalpy of reaction is called as thermochemical equation. Expert Solution & Answer Want to see the full answer? Check out a sample textbook solution See solution chevron_left Previous Chapter 6, Problem 6.57QP chevron_right Next
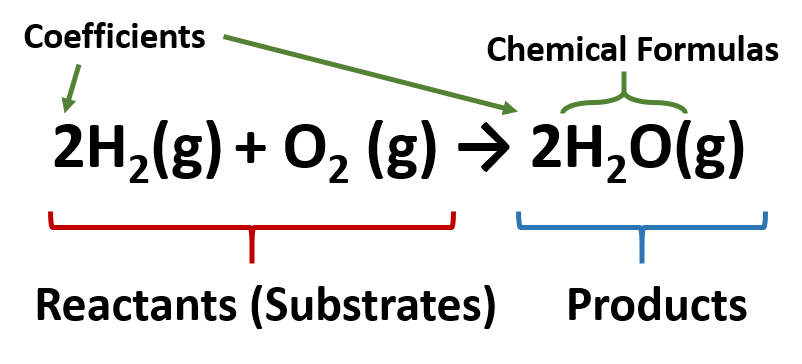
What are Chemical Equations? Detailed Explanation, Examples The reactants and the products (for which the chemical formulae are written in chemical equations) can be separated by one of the following four symbols. In order to describe a net forward reaction, the symbol '→' is used. In order to describe a state of chemical equilibrium, the symbol '⇌' is used.
Chemical equations Flashcards | Quizlet Terms in this set (17) chemical reactions. shows chemical changes. reorganization of the atoms in one or more substances. Product. says what it is, a product. yield sign. to yield, producing, resulting in a product. States of matter. solids (s), liquids (l), gas, (g), aqueous (aq)
In the equation H2 + O2 arrow H2O, the 2 in the subscript after ... In the equation H2+O2→H2O H 2 + O 2 → H 2 O , the 2 in the subscript after H H is called the phase label. Is this statement true or false? Explain. Chemical ...
Factor-Label Method in Chemistry: Definition, Examples & Practice ... The Factor-Label Method. Believe it or not, one simple method can be used to accomplish many of the basic calculations in chemistry. The method does not involve years of calculus courses or other ...
Laboratory evaluation of alum, ferric and ferrous-water treatment ... Web27.08.2020 · After the adsorption phase qe's were calculated , the data were fitted to determine the kinetic coefficients. This was only possible for the adsorption phase as desorption phase with surface water introduced new PO 4 at each refill step. The data was evaluated with both the Langmuir and Freundlich equations models.
Solved 1. Write balanced chemical equations using proper | Chegg.com 1. Write balanced chemical equations using proper phase labels for these reactions: (you may also handwrite in the equations) a) Iron metal with oxygen gas to produce solid iron (III) oxide, also known as rust.
Phase Transitions: Melting, Boiling, and Subliming Chemical equations can be used to represent a phase change. In such cases, it is crucial to use phase labels on the substances. For example, the chemical equation for the melting of ice to make liquid water is as follows: H 2 O (s) → H 2 O (ℓ) No chemical change is taking place; however, a physical change is taking place. Heating Curves




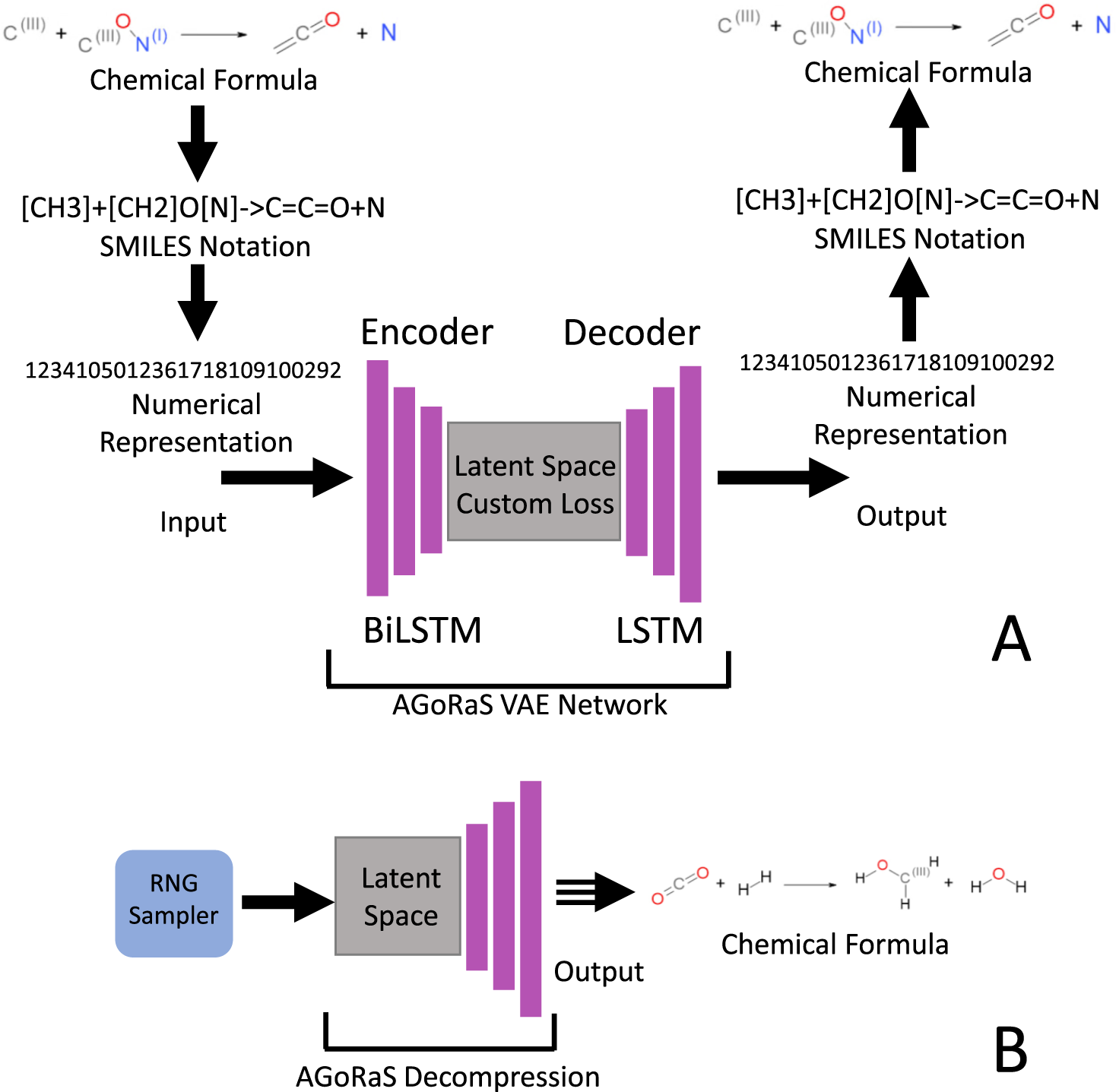


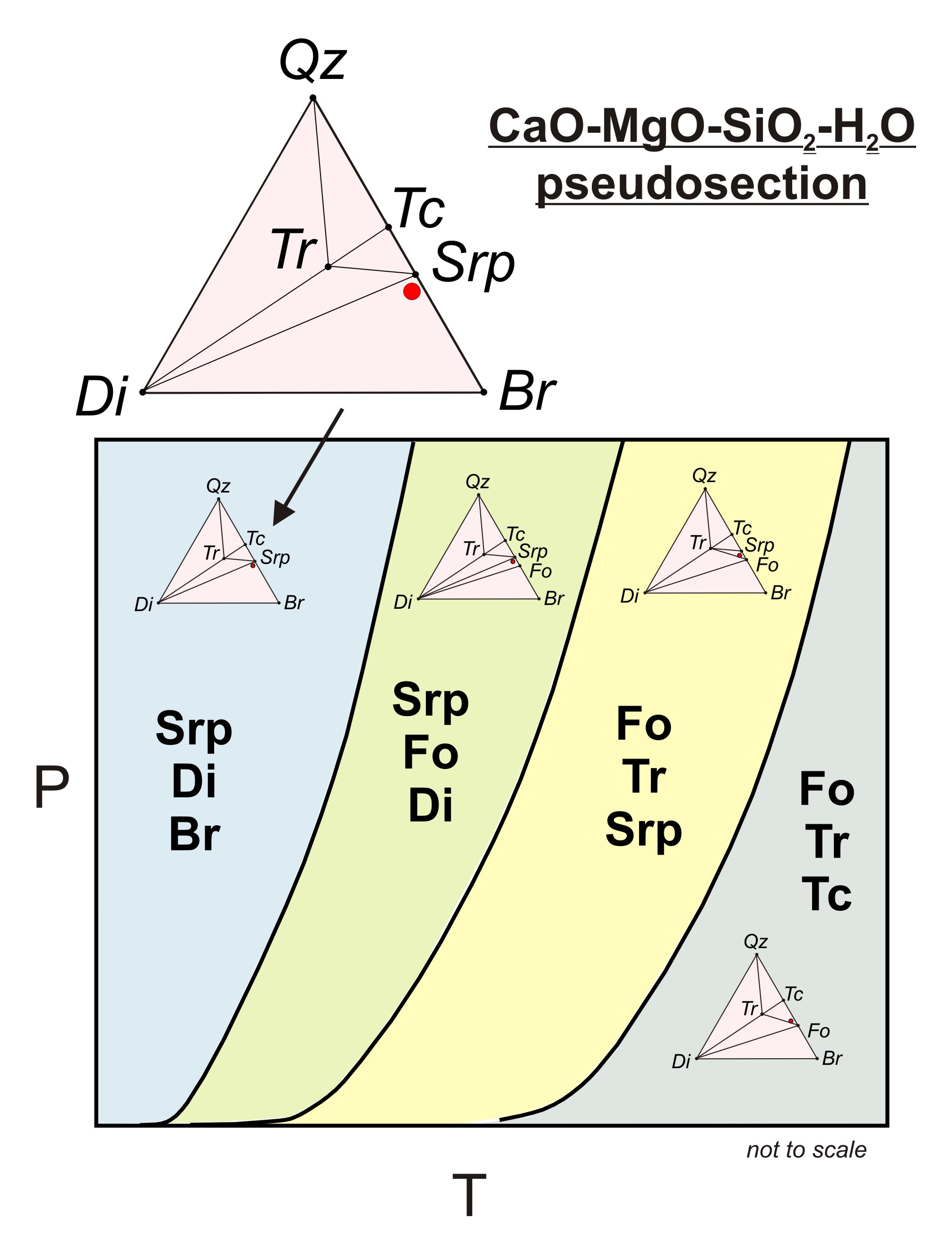

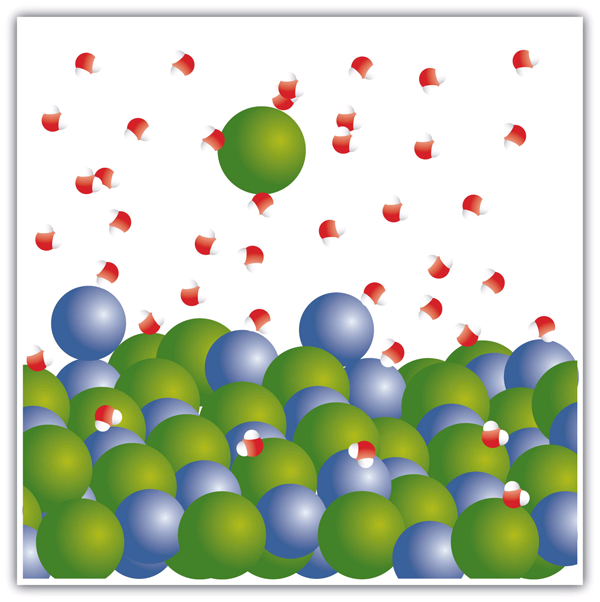
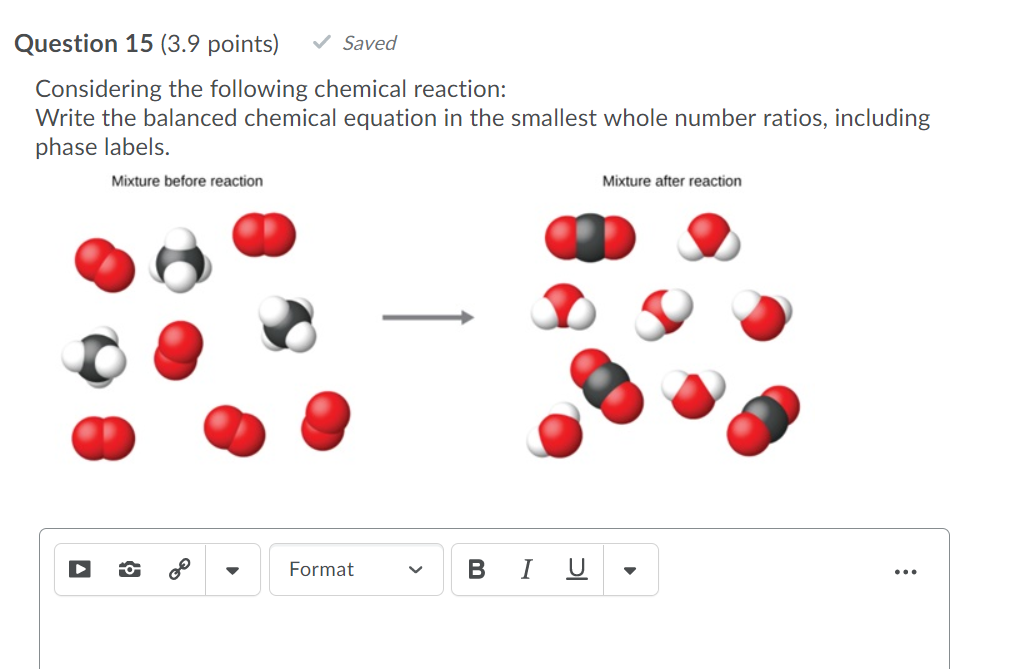












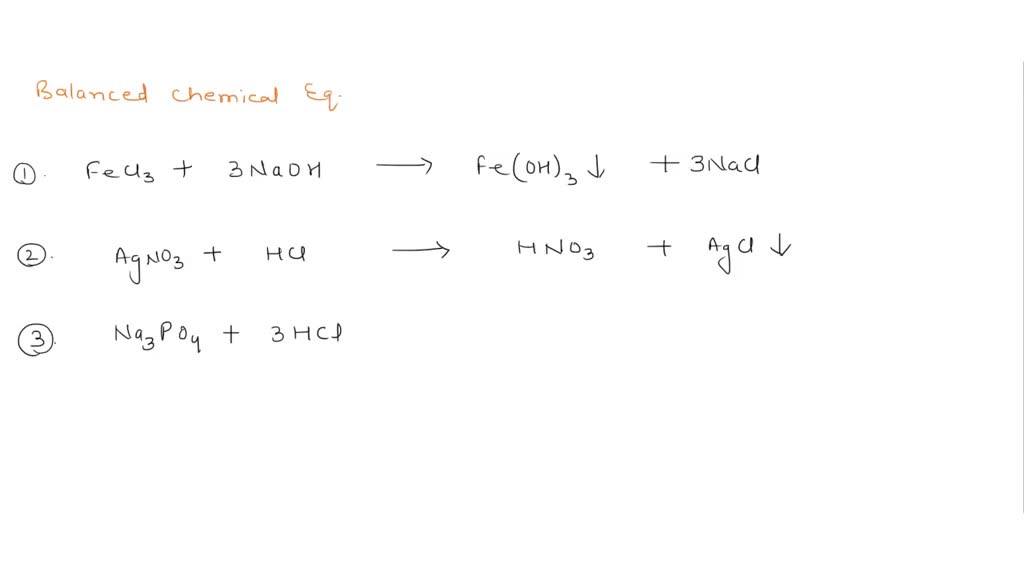

Post a Comment for "45 phase labels in chemical equations"